– Guest Blog Post by Danika

I was 19 years old when I had my first contact with melanoma. I had a beauty mark on my back and it began to grow bigger and had a reddish hue.
A dermatologist did a biopsy and I had the diagnosis on December 28 2011; I was 19 and I had a malignant melanoma, the most serious form of skin cancer. I had a PET scan and a lymphoscintigraphy. Fortunately, at that time, I had no metastases. I had an operation to remove the beauty mark and the skin below and I was told that everything was fine. I only kept a scar shaped like a bird as a souvenir.
However, two years later, I was stage four melanoma.
The previous weeks, some lumps grew on my abdomen, my chest, and even my face. So we did biopsies and it was subcutaneous metastases. A PET scan later, I learned that I also had metastases to the liver, lungs and bones. At that time, I knew it was serious, but I had no idea how my life was about to change.
I was quickly supported by my hospital, the CHUM Notre-Dame, who proposed me to be part of a research protocol. I started taking tablets of « LGX818 », it was so new that they did not even have an official name yet. They were anti-BRAF drugs, since I had the BRAF mutation. One of the worst side effects that I had was peripheral neuropathy. It felt like having electrical shocks in my body. Then came the first scans results ; the treatment was working since some metastases decreased volume and no new one had appeared. Muscle pain, nerve, joint, skin problems, all of it was now worth it.
However, a few weeks after starting treatment, a new side effect appeared. In medical parlance, it is called “alopecia”. More simply, it means hair loss. Of course when you think chemotherapy you think hair loss, but my treatment was not supposed to do that, so I wasn’t prepared. In a few weeks I had to witness the loss of at least 50% of my hair, and believe me, I had a lot of hair! I found some in my bed, in the shower, on the couch, carpet, everywhere … I couldn’t take it anymore so I asked my mother to shave all of it. I anticipated this moment and yet none of us has shed tears. To my surprise, I was not so bad without hair. I still decided to wear a wig to school, to avoid passing from one extreme to the other and catch the eye of everyone.
After nearly 8 months of treatments, I had an appointment with my oncologist and I felt that something was wrong. Indeed, the latest scan results showed new lesions. My body got used to the medication and became resistant to it. We had to change treatment. New drugs were just emerging in Canada and were offering promising results. However, we must pass through a conventional chemotherapy before gaining access to the other treatments. These new treatments cost a lot of money so you have to « try everything » before.
A week later, it was time for that chemotherapy called Dacarbazine. It was the first time I found myself in these kinds of rooms, you know the rooms where there are several chairs next to each other, and on which are literally plugged patients. And of course, most patients are older and the majority have no hair. The image gives a shock. However, once installed in my chair, I noticed that the atmosphere was not so bad.
The next days were very difficult. I had nausea, fatigue and flu-like symptoms. I was going to school when my condition would let me. My next dose was scheduled three weeks later but it never took place. Bumps had appeared on my body; Dacarbazine wasn’t working on me at all. At least, now I had access to the new treatment. Again, I had to change course.
The new treatment, Yervoy, was actually immunotherapy, which rely on our natural immune system to destroy cancer cells, that’s the essence of immunotherapy. A few days later, on May 14, the day before my birthday, I found myself once again in the treatment room, but this time with another liquid pouring into my veins.
The first shot was held pretty well and I could finish my session. In addition, hair began to appear on my skull. However, bad news came back; I started to feel horrible abdominal pain. I even felt my tumors growing back. On the abdomen, I had a big lump like a cherry, right under my skin. So I felt it constantly.
I went to the hospital and the on-call oncologist decided to do the scans and all the tests right then. She came back several hours later with the results … Just by the look on his face, his way of coming to me, I felt it was bad. He sat on my bed, took my hand and said: “The news are not good, tumors got bigger, especially the one on the liver and it’s probably what’s causing your pain. ”
So … we turned to a fourth type of treatment in one year. I was exhausted. This was the latest treatment available in Canada for melanoma. It was still immunotherapy, but called Pembrolizumab (Keytruda).
The first Keytruda treatment went well. A few days later, the pain seemed to lessen a bit. I got to do some activities, saw some friends, etc. A few weeks later, the scan results finally brought some good news; after only 3 shots, metastases were reduced by approximately 50%! And I had almost no side effects; only fatigue the days following treatment, as well as vitiligo that appeared on my body.
So here I am, May 2016, I just turned 24 years two days ago, and my current treatment, pembrolizumab, is finally working. I had 13 shots so far and I will continue for at least another year. I hope that future scans will be even better than the last ones. I got back to school, my hair grew back and I can finally start to enjoy life again!
Those last two years were a rollercoaster of emotions. We all have the right, at times, to be afraid and desperate. The important thing is to get up and always keep in the back of our mind the hope to believe that everything will be fine.
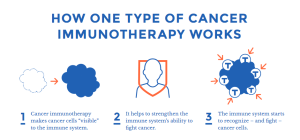 “Redefining Cancer Treatment” is an apt title for the topic: what is cancer immunotherapy, and how does it work, exactly? These questions are answered in a few pages and a downloadable infographic, all in language that is easy for patients and their caregivers to understand.
“Redefining Cancer Treatment” is an apt title for the topic: what is cancer immunotherapy, and how does it work, exactly? These questions are answered in a few pages and a downloadable infographic, all in language that is easy for patients and their caregivers to understand. Merck also has an interactive website listing enrolling clinical trials for their treatments, in cancer and in other indications. Click HERE to view the site and search for your disease type: Merck Clinical Trials
Merck also has an interactive website listing enrolling clinical trials for their treatments, in cancer and in other indications. Click HERE to view the site and search for your disease type: Merck Clinical Trials