Clinical Trials in Canada
New treatments are tested in clinical trials before they are approved for general use. There are safeguards in place to ensure clinical trials are as safe as possible and meet medical ethical standards. Participating in a trial can be a way to have access to potentially helpful new therapies you couldn’t get otherwise.
Clinical trials are funded by pharmaceutical companies evaluating their new treatments. Therefore, the treatments, tests, and doctor visits are usually paid for and patients are followed carefully. Clinical trials usually have very specific criteria for the patients who can participate, such as severity or stage of disease and whether and what types of previous treatments you have had.
If you are found to be eligible, most studies will not allow you to choose whether you will be put into the group of patients given the existing standard treatment or the group receiving the new medicine. Often, neither you nor your doctor will be told which treatment you are receiving. This randomization of what you are assigned to, and blinding of you and your doctor to the treatment you are getting, is an important part of ensuring clinical trials are as free from bias as possible and ensures the results are as clear as possible.
If you are interested in participating in a clinical trial, ask your doctor if there are any appropriate studies available to you. We have also included a list of Canadian databases so you can search for trials on your own, but please be aware that you may not be eligible for all options presented to you and that these databases might not be up-to-date.
Watch The Basics about Clinical Trials with Dawn Richards, PhD
Saskatchewan
Manitoba
Nova Scotia
Prince Edward Island
Newfoundland and Labrador
Yukon and Northwest Territories and Nunavut*
*Please see Canada-wide clinical trial databases.
Finding Global Clinical Trials
The following websites might also assist you in finding global clinical trials for melanoma or other cancers:
ClinicalTrials.gov is a global database of both private and public clinical trials. It is complied and maintained by the U.S. Library of Medicine.
Leal Health is a tool made for patients who are looking for clinical trials. Their technology analyzes all relevant treatment options and instantly presents only what is a match for your condition in easy to understand language. They remove barriers to becoming a true partner with your oncologist in making decisions about your future.
Interactive map searching tool to find enrolling clinical trials in Canada for any health indication, including melanoma and non-melanoma skin cancers.
ForPatients includes a search tool for finding clinical trials in various cancer indications, plus other medical conditions such as autoimmune or neurodegenerative disorders. It contains detailed information about clinical trials in general, discusses patients’ rights in and after a clinical trial, and points out where to find published results of any given Roche trials.
Watch Jurisdictional Roles in Canadian Drug Access and the Impact of Social Determinants of Health with Louise Binder
Dr An-Wen Chan (University of Toronto) on Clinical Trials in Canada
Information about Clinical Trials
Phases are the sequential waves of patients in clinical trials, in order to test the treatment on a wider variety of subjects and to prove endpoints. More information on each phase is found below.
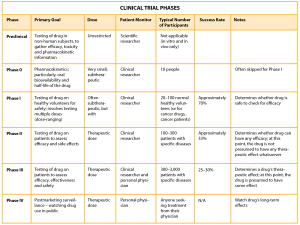
Table source: Save Your Skin Foundation
Phase 1: These trials are also sometimes called ‘first in humans’ because it is usually the first time the intervention is tested in people. These trials aim to establish the safety, dose and side effect information of the intervention in a small number of participants (usually 20-80 people).
Phase 2: These trials aim to ensure the intervention does what it is supposed to do and to determine if the dose should be changed, and to continue to learn about potential side effects. This phase is carried out in a larger number of participants (about 100-300 people).
Phase 3: These trials are ‘randomized clinical trials’ and test how long the effects of an intervention last, and continue to learn about potential side effects. There are a large number of participants in this phase (usually 1000s of people). You can read more about randomization below.
Phase 4: These trials are also called post-marketing surveillance because they involve monitoring an intervention after it is being sold (that is, on the market). This phase studies long term effects and any side effects on a very large group of people.
The following video from the National Cancer Institute (NCI) describes clinical trial phases. Please be aware that the NCI is an American institution, and therefore not all of their more specific content will be applicable to Canada.
While you will not be asked to pay to participate in a trial, there may be costs associated with your hospital visits (meals, transportation, parking), especially if you are travelling a larger distance for treatment. You may also need to take days off of work in order to receive treatments or if you experience side effects; depending on these side effects, additional medications or types of care may or may not be covered by your insurance.
- Principal Investigator – The person responsible for the study and may be a physician or a dentist or another health professional.
- Clinical research coordinator – The individual who is responsible for the day-to- day coordination of the clinical trials including planning and scheduling of participants and collecting data. They are often the person that participants interact with the most on the study team.
- Other research site staff may include:
- Research nurse – A nurse who is part of the research team, and may also be a clinical research coordinator for the study.
- Genetic counsellor – A healthcare provider with an education in both genetics and counselling.
- Social worker – A professional who helps people solve and cope with challenges they face in their lives.
- Dietitian – An individual educated about nutrition and its relation to health.
- Pharmacist – A healthcare professional who works with medicines and provides them to individuals with a medical prescription and who can also provide counselling services related to medicines.
- Nurse practitioner – A nurse who also has an advanced degree and other clinical training, and is often allowed to provide primary care and other medical services to patients.
- Patient partners – People who live or have lived with a health condition or who have an experience in the healthcare system, and who bring this perspective to the clinical trial team.
The following video from the National Cancer Institute (NCI) outlines the precautions taken to ensure patient safety throughout the trial. Please be aware that the NCI is an American institution, and therefore not all of their more specific content will be applicable to Canada. The Canadian equivalent of the institutional review board (IRB) is the research ethics board (REB).
From Clinical Trials Ontario:
There are some things that you should look for in helping you determine if a clinical trial is a good clinical trial for you, and may include:
- If you meet the eligibility criteria to participate in the clinical trial. In order to be accepted to participate in a clinical trial, you must meet certain criteria.
- If you are asked to pay to participate in a clinical trial. While you may be required to pay for expenses such as traveling to your appointments, parking, etc., you should not be asked to pay to participate in the clinical trial.
- The qualifications of the clinical trial team. Often people on the clinical trial team have worked in or studied the area of the clinical trial or have an affiliation with an academic institution such as a university.
- If the clinical trial application was reviewed by a regulatory agency. Examples of regulatory agencies are Health Canada and the US Food and Drug Administration. Health Canada’s role is to ensure Canadians have access to safe and effective drugs and health products, and they review all clinical trial applications.
- If the clinical trial was reviewed and approved by a research ethics board ( also called “REB” for short). The REB reviews proposed clinical trials with an aim to make sure they are acceptable from a Research Ethics Board perspective.
- If the clinical trial was reviewed and approved by the institution or organization (e.g. hospital) where the clinical trial is taking place.
You can choose to end your participation in the clinical trial at any time without having to provide a reason – this is called withdrawal. If you choose to withdraw from the study, you are encouraged to contact the study doctor or study staff to let them know your decision or to ask them further questions before you make a decision.
If you do withdraw, you may be asked questions about your experience with the intervention, and to have laboratory tests and physical examinations considered necessary to safely stop your participation.
You may also be able to withdraw your permission to use information that was collected about you for the clinical trial and this would mean that you withdraw from the clinical trial.
The following video from the National Cancer Institute (NCI) offers advice on determining whether a clinical trial is for you. Please be aware that the NCI is an American institution, and therefore not all of their more specific content will be applicable to Canada.
The following terms are sourced from Clinical Trials Ontario.
For a more extensive glossary of terms, including different treatment types, see the Save Your Skin glossary of melanoma terms.
Adverse Event (also called reaction/side effect)
An unintended medical event that may happen during a clinical trial and that may or may not be expected and may or may not be due to the intervention(s) being tested. Adverse events can be mild or less serious (for example a rash) or more severe or serious (for example, death). All adverse events that happen during a trial are reported whether or not they are caused by the intervention. For some people, considering potential adverse events is important when they are thinking about participating in a clinical trial.
Arm
A group of participants in a clinical trial that receive a specific intervention (or no intervention) based on the protocol of the clinical trials.
Bias
In a clinical trial, this is a flaw in the study design or in how information is collected or interpreted. A bias may lead to incorrect conclusions being drawn from the study.
Blinded
If a trial is “blinded” one or more individuals do not know what treatment group participants are randomized to. When participants are “blinded” they do not know which intervention they are receiving. A “double-blinded” study is when both the participants and the study team do not know which treatment arm the participants are in. Blinding is used to help ensure the assessment of outcomes (e.g., disease status, side effects) of a trial are not biased by people knowing what treatment participants receive.
Efficacy
Another word for effectiveness, which means the ability of an intervention to produce a desired effect or do what it is supposed to do.
Eligibility Criteria
The ‘qualifications’ set that determines whether or not the trial is right for you. These qualifications may include a disease or condition, a certain age range, living in or near a specific location, a specific ethnicity, etc. Inclusion criteria are the characteristics needed to be part of the clinical trial. For example, for a cancer clinical trial, this might be specific genetic characteristics. Exclusion criteria are characteristics that don’t allow participation in the clinical trial, for example, ‘pregnancy’ or a specific health condition.
Endpoint
A result that is measured and is used to see whether the clinical trial intervention is beneficial or not. Some examples may include whether a participant contracted a disease, how long a participant survived after receiving a treatment, etc.
Expanded Access
Sometimes also called compassionate use. This is a way for patients to access interventions that are not yet approved by a regulatory agency, and in circumstances where there are not comparable or satisfactory treatments options available outside of a clinical trial.
Informed Consent
Participation in a clinical trial is voluntary. If you are thinking about participating in a clinical trial it is important that you are provided with enough information to make an informed decision, an opportunity to ask questions and talk to others you may choose to (e.g. your family or friends) and the time to make a decision that is right for you.
A key part of the informed consent process for most clinical trials is a document called the ‘informed consent form’ or ‘consent form’. This document should provide all relevant information about the clinical trial and be written in a way that is understandable to you. You should be given enough time to read it and ask any questions you may have. If you agree to participate, you may be asked to sign the consent form and should be provided with a copy of the form for your own keeping.
Informed consent does not always need to be provided in a written consent form. It may be provided in another, non-written form, and however this happens, it is always approved by a Research Ethics Board.
Informed consent is an ongoing process throughout a clinical trial. You can withdraw your consent to participate at any time. If you are in a clinical trial and your doctor or study team learn new information that might affect your willingness to continue to participate (e.g., new safety information about the intervention), you should be provided with this information.
Intervention
Clinical trials study different treatments or interventions on their own or in combination with others. Different intervention types studied in clinical trials may include:
- a drug such as a pill, an intravenous treatment, etc.
- a medical device such as a pacemaker or an insulin pump
- a type of surgery
- radiation therapy
- a diagnostic procedure
- a diet and lifestyle change such as exercise.
Masking
Another word for blinding
Open Label Extension
An extension to a blinded placebo-controlled clinical trial whereby all participants receive the active study drug or treatment, no matter which study treatment arm they started in. When the extension trial starts, participants and the study team are considered ‘unblinded’ since they know all participants are receiving the active study drug or treatment (vs. the placebo).
Outcome
A characteristic(s) being measured in a clinical trial that is related to health. Some examples may include disease status (e.g., if the disease being studied gets worse or better), survival status (how long participants live), health status (fatigue or pain) and side effects.
Placebo
A clinical trial protocol contains detailed documents with information on how and why the trial is being done, how the trial will be done, how the results will be gathered, analyzed and shared, and who is involved. The typical parts of a clinical trial protocol are:
- Background scientific and clinical information
- Justification on why the trial is important
- Key measurements or endpoints of the trial
- Who can and can’t be in the clinical trial also called the eligibility and ineligibility criteria
- What treatments will be used and how, and how or if any adjustments to treatment will be made
- The assessments that will be undertaken, including what kind, when, and the type of assessment (for example, blood tests, doctors visits, questionnaires, etc.)
- A sample informed consent form
- The number of participants needed and why
- How results will be analyzed and shared
- Safety reporting information
- Data that will be collected.
Protocol
A clinical trial protocol contains detailed documents with information on how and why the trial is being done, how the trial will be done, how the results will be gathered, analyzed and shared, and who is involved. The typical parts of a clinical trial protocol are:
- Background scientific and clinical information
- Justification on why the trial is important
- Key measurements or endpoints of the trial
- Who can and can’t be in the clinical trial also called the eligibility and ineligibility criteria
- What treatments will be used and how, and how or if any adjustments to treatment will be made
- The assessments that will be undertaken, including what kind, when, and the type of assessment (for example, blood tests, doctors visits, questionnaires, etc.)
- A sample informed consent form
- The number of participants needed and why
- How results will be analyzed and shared
- Safety reporting information
- Data that will be collected.
Randomized
Another way of saying you get there by chance. A common type of clinical trial is a “randomized” clinical trial. These are typically called “phase 3 trials”. In randomized trials two or more interventions (or treatment arms) are being compared to each other. When a trial is randomized, participants are assigned by chance to one of the interventions (or treatment arms). This is important in helping to ensure the results of the trial are not due to health professionals or participants choosing what treatment arm they want to participate on.
Research Ethics Board (REB)
An independent committee of people with different expertise (for example, law, medicine, ethics, community, etc.) that reviews the ethical acceptability of research involving humans. The REB reviews all study materials including protocols and information or materials given to potential or actual participants with a view to ensuring participants are properly informed and free to make a voluntary decision about participating, and that a study’s potential risks are balanced with potential benefits. The REB continues to review information about the study as long as it is ongoing, including any adverse events and changes to the study along the way.
Sponsor
The term sponsor is used in two ways in Canada. Health Canada considers the ‘sponsor’ of the trial the organization or individual that is responsible for the regulatory submission and overall conduct of the trial. This can be a company, a hospital, a research group or a clinician researcher. The word sponsor is also used in Canada to describe who is funding the clinical trial. This might be pharmaceutical or medical device company, for example. This same company may or may not be the regulatory sponsor with Health Canada.
Withdrawal
You can choose to end your participation in the clinical trial at any time without having to provide a reason – this is called withdrawal. If you choose to withdraw from the study, you are encouraged to contact the study doctor or study staff to let them know your decision or to ask them further questions before you make a decision.
If you do withdraw, you may be asked questions about your experience with the intervention, and to have laboratory tests and physical examinations considered necessary to safely stop your participation.
You may also be able to withdraw your permission to use information that was collected about you for the clinical trial intervention.
The study doctor may stop your participation in a study early, and without your consent, for reasons such as:
- The study intervention does not work for you
- You are unable to tolerate the study intervention
- You are unable to complete all required study procedures
- New information shows that the study intervention is no longer in your best interest
- The study doctor no longer feels this is the best option for you
- The Sponsor decides to stop the study
- The Regulatory Authority/ies (for example, Health Canada) or research ethics board withdraw permission for this study to continue
- Your group assignment becomes known to you or others (like the study doctor or study staff)
- If you are female and plan to or become pregnant
If this happens, it may mean that you would not receive the study intervention for the full period described in this consent form.
If you are removed from a study, the study doctor will discuss the reasons with you and plans will be made for your continued care outside of the study.
Other Resources
SYSF:
- Webinar: Understanding the Drug Approval Process: From Clinical Trials to Public Reimbursement
- Wednesday, April 19, 2023 at 8pm EST | 6pm MDT | 5pm PST with Dawn Richards, PhD, Director of Patient and Public Engagement at Clinical Trials Ontario, Founder of Five02Labs & Louise Binder, Lawyer, Senior Health Policy Expert at Save Your Skin Foundation
- Listen to a recording of this webinar here
- Webinar: Clinical Trials: What Patients Need to Know
- Thursday, February 9, 2017 at 2pm EST | 12pm MDT | 11am PST with Dr. Natasha Kekre, Associate Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute
- Listen to a recording of this webinar here
Other sources:
- Canadian Cancer Society “Clinical Trials” page
- Clinical Trials Ontario
- Cancer.Net “About Clinical Trials” page (please be aware that cancer.net is an American group and not all information on this page will be directly applicable to Canada)
- AIM at Melanoma “Clinical Trials” page (please be aware that AIM at Melanoma is an American group and not all information on this page will be directly applicable to Canada)
- Melanoma Research Alliance “Clinical Trials” page (please be aware that the Melanoma Research Alliance is an American group and not all information on this page will be directly applicable to Canada)
Offered a clinical trial? Here are questions to ask.
Click the image above for the full question checklist, or click here for a printable version of this checklist.



