Immunotherapy is the latest advance in the way cancer is treated, using the body’s own immune system to fight otherwise deadly disease. Meloney Edghill knows it can work.

Meloney also knows what the mother of a young child thinks about when told she has terminal cancer. She chokes back tears remembering: “I was worried it would kill me before my 4-year-old son would have any memories of me. I didn’t want him growing up and not remembering me at all.”
That was in April 2006. The fact that Meloney is still here to tell her story – and watch her son grow to a teenager – is thanks to the development of the newest form of cancer treatment, immunotherapy. Unlike the traditional approaches to cancer treatment – surgery, radiation, chemotherapy and targeted therapy – immunotherapies are drugs that release the natural brakes on the body’s own immune system so it can fight and kill the cancer cells.
In April 2006, the young mother was living in Edmonton and had a lump growing on the front of her shoulder. When she went to the doctor to check it out, it was too late. She was diagnosed with metastatic melanoma, the most serious form of skin cancer. It accounts for just 8 per cent of skin cancer cases, but is responsible for 70 per cent of deaths from the disease.
When she was first told she had melanoma, Meloney didn’t realize the implications. “I initially thought they would just cut it out and everything would sort of be OK after that,” she recalls. “We found out very fast that it was not that simple and in fact there were very few options. We were devastated.”
At the time, the average life expectancy for someone with Meloney’s diagnosis was about six to nine months. That October, she enrolled in a trial of a new melanoma immunotherapy that was in an early study.
In January 2007, when tested to see how she had responded, Meloney was taken by surprise. “All of my cancer was just about gone after that,” she says. “It was unbelievable to my doctors and nurses that something had worked that well and that quickly.” According to her doctor, today Meloney is cancer free and her son, who is a now a teenager, has grown up with his mother at his side.
Meloney’s experience with the treatment is not shared by all melanoma patients, but the immunotherapy she took has shown good results in studies. It has been approved for use in Canada and other countries and is the first-ever treatment shown in clinical studies to improve survival of patients with metastatic melanoma. Ongoing research continues to give new hope to those diagnosed with the disease.
Today there are even newer immunotherapies available. The latest ones work in different ways to stimulate the immune system, shutting off a different one of the “checkpoint inhibitors” which act as natural brakes on the immune system and prevent it acting against cancer cells. These newer immunotherapies are also being studied with promising results in combination with the older drugs in melanoma and as potential treatments for a wide variety of other cancers and are showing promising results.
“Immunotherapy has completely transformed the way advanced melanoma is treated. Just a few years ago patients who were diagnosed were desperate and many were told to ‘get their affairs in order’. In just a short period this cancer went from being defined as a deadly disease to a cancer that patients may be able to survive,” says Kathy Barnard, Founder and President of the Save Your Skin Foundation, a melanoma patient support organization that also aims to educate the public on the importance of protecting the skin from the sun’s harmful ultraviolet rays, a major risk factor for skin cancer.
“What’s even more exciting is that there is ongoing research with immunotherapy which means more treatments are available to patients to give them options. Can you imagine? Advanced melanoma patients have treatment options to survive? I never dreamed this would happen in my lifetime. I’m here today witnessing history being made. In fact, I’m living proof of it,” Kathy adds. She herself is a melanoma survivor, another beneficiary of the same treatment that helped Meloney. Kathy has since devoted herself to helping others and educating about the disease.
Copyright 2015 ZoomerMedia Limited




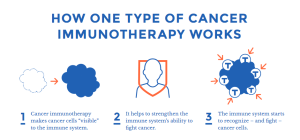 “Redefining Cancer Treatment” is an apt title for the topic: what is cancer immunotherapy, and how does it work, exactly? These questions are answered in a few pages and a downloadable infographic, all in language that is easy for patients and their caregivers to understand.
“Redefining Cancer Treatment” is an apt title for the topic: what is cancer immunotherapy, and how does it work, exactly? These questions are answered in a few pages and a downloadable infographic, all in language that is easy for patients and their caregivers to understand.