***
While we’re finishing up our report on the ASCO Annual Meeting 2018 in Chicago, we’d like to share with you some resources about trials that were discussed at an ASCO satellite meeting: Best of ASCO 2018 Montréal, which took place in Montréal, QC on June 19th, 2018. From 7:50-8:20 pm, Dr. Wilson Miller (McGill) gave a talk entitled “Best of Melanoma,” which highlighted some of the notable melanoma studies discussed at the ASCO annual meeting.
For those interested in more ASCO recaps, Oncology Education has posted video resources, including Dr. Jeffrey Weber (NYU) discussing Checkmate 238, Dr. Max Madu (Netherlands Cancer Institute) on the 8th AJCC melanoma staging system, and a roundtable discussion of ASCO highlights with Dr. Marcus Butler (Princess Margaret Cancer Centre), Dr. John Walker (Alberta Cancer Centre), and Dr. Jason Luke (University of Chicago). These videos are available here, though you will need to register for the Oncology Education website to view them.
Below is a list of some of the trials Dr. Miller presented, with links to the ASCO abstracts for further reading:
Abstract number 9501: “Final analysis of DeCOG-SLT trial: Survival outcomes of complete lymph node dissection in melanoma patients with positive sentinel node.”
Presented at ASCO Annual Meeting, Chicago, in the Melanoma/Skin Cancers Oral Abstract Session (Monday, June 4, 8:00-11:00 AM).
Authors: Ulrike M. Leiter et al.
Retrieved from: http://abstracts.asco.org/214/AbstView_214_216115.html
The DeCOG-SLT trial assessed whether complete lymph node dissection can result in increased survival compared with observation in patients with positive sentinel node biopsy in a randomized phase III trial. The update presented at ASCO 2018 comes three years after the inclusion of the last patient. After the median 72-month follow-up time, there seemed to be no survival benefit in patients with positive sentinel node biopsy with complete lymph node dissection, compared to observation. More information about the methods and results of the study can be found at the link above.
Abstract number 9503: “4-year Survival and Outcomes After Cessation of Pembrolizumab (pembro) after 2-years in Patients (pts) with Ipilimumab (ipi)-naive Advanced Melanoma in KEYNOTE-006 [NCT01866319]”
Presented at ASCO Annual Meeting, Chicago, in the Melanoma/Skin Cancers Oral Abstract Session (Monday, June 4, 8:00-11:00 AM).
Authors: Georgina V. Long et al.
Retrieved from: http://abstracts.asco.org/214/AbstView_214_222303.html.
The KEYNOTE-006, or NTO1866319, sought to establish the efficacy of pembrolizumab over ipilimumab in advanced melanoma. The data includes four year outcomes, long term data for patients who have completed two years of pembro, and data for second course. The results suggest that pembrolizumab can provide durable anti-tumour activity in treatment-naive or previously treated patients. 86% of the patients who had completed two years of pembro were progression free at 20 months. The data suggests that pembro is safe, and can be used as a second-course treatment to provide additional anti-tumour activity. For more information, see the link above.
Abstract number 9594: “Assessing the Value of Nivolumab (NIVO) versus Placebo (PBO) and Ipilimumab (IPI) as Adjuvant Therapy for Resected Melanoma [EORTC 18071]”
Presented at ASCO Annual Meeting, Chicago, in the Melanoma/Skin Cancers Oral Abstract Session (Monday, June 4, 8:00-11:00 AM).
Authors: Morganna Louise Freeman et al.
Retrieved from: http://abstracts.asco.org/214/AbstView_214_220221.html.
This study assessed the cost of cancer therapies in the context of clinical benefits. Data from CheckMate 238 and EORTC 18071 (nivolumab) was compared to placebo and ipilimumab in the adjuvant setting for patients with resected melanoma was used to consider the cost for each recurrence-free life month (RFLM) and associated medical costs. They found that nivo has a lower medical cost per RFLM than both placebo and ipi in patients with resected IIIB and IIIC cutaneous melanoma, and has superior drug costs per RFLM relative to placebo and ipi over eighteen months. Data suggests that follow-up will continue to determine the cost-effectiveness of adjuvant NIVO. For methods, results, and outcome measures, see the link above.
Abstract number 9502: “Adjuvant Therapy with Nivolumab (NIVO) versus Ipilimumab (IPI) After Complete Resection of Stage III/IV Melanoma: Updated Results from a Phase III Trial (CheckMate 238)”
Presented at ASCO Annual Meeting, Chicago, in the Melanoma/Skin Cancers Oral Abstract Session (Monday, June 4, 8:00-11:00 AM).
Authors: Jeffrey S. Weber et al.
Retrieved from: http://abstracts.asco.org/214/AbstView_214_214567.html.
With a minimum follow-up of 18 months, the initial report data from the CheckMate 238 trial demonstrated that nivolumab had longer recurrence-free survival over ipilimumab in patients with resected stage III or IV melanoma. At ASCO, phase III data with an additional six months of follow-up was reported. With this extended follow-up, nivo continued to demonstrated a sustained benefit versus ipi for patients with resected stage III/IV melanoma at a high risk of recurrence, PD-L1 expression, or BRAF mutation. More information can be retrieved at the link above.
Abstract number 9514: “Phase II Trial of Pembrolizumab (pembro) plus 1 mg/kg Ipilimumab (ipi) Immediately Following Progression on Anti-PD-1 Ab in Melanoma (mel)”
Presented at the ASCO Annual Meeting 2018, Chicago, in the Melanoma/Skin Cancer Poster Session (Monday, June 4, 1:15-4:45 PM).
Authors: Daniel Olsen et al.
Retrieved from: http://abstracts.asco.org/214/AbstView_214_215997.html.
This study sought to examine the role of the immunotherapy anti-PD-1 + CTLA-4 combination after the first line anti-PD-1. They are reporting the first potential data examining pembrolizumab + low dose ipilimumab following progression on anti-PD-1. The results suggest that low dose ipi + pembro is tolerable and has anti-tumour activity in melanoma patients who have progressed on an anti-PD-1 immediately prior. For more information on this abstract and ongoing trial, see the link above.
Abstract number 9542: “BRAF/MEK Inhibition in Melanoma Patients with Rare BRAF Mutations”
Presented at the ASCO Annual Meeting 2018, Chicago, in the Melanoma/Skin Cancer Poster Session (Monday, June 4, 1:15-4:45 PM).
Authors: Jessica Cecile Hassel et al.
Retrieved from: http://abstracts.asco.org/214/AbstView_214_226845.html.
This study uncovered efficacy data for BRAF/MEK inhibition, which is standard care for patients with BRAF V600E/K mutated melanoma. The results suggest that patients with tate BRAF mutations often respond to targeted therapy. Less likely to respond to BRAFi monotherapy are patients with non-V600 mutations, but MEKi as monotherapy or combined with BRAFi seems more promising for these patients. For more information, including a data breakdown, see the link above.
We hope this information was interesting and helpful– stay tuned to our social media channels for study news, and our upcoming ASCO Annual Meeting 2018 report!






 Save Your Skin Foundation openly discusses the need for access to these treatments by melanoma patients across Canada, and we jumped on the opportunity to be the primary patient group sponsor of this feature, sharing our collective patient experience with these therapies. On
Save Your Skin Foundation openly discusses the need for access to these treatments by melanoma patients across Canada, and we jumped on the opportunity to be the primary patient group sponsor of this feature, sharing our collective patient experience with these therapies. On 


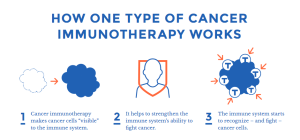 “Redefining Cancer Treatment” is an apt title for the topic: what is cancer immunotherapy, and how does it work, exactly? These questions are answered in a few pages and a downloadable infographic, all in language that is easy for patients and their caregivers to understand.
“Redefining Cancer Treatment” is an apt title for the topic: what is cancer immunotherapy, and how does it work, exactly? These questions are answered in a few pages and a downloadable infographic, all in language that is easy for patients and their caregivers to understand.